Background: Classic Hodgkin Lymphoma (CHL) is a B-cell lymphoma characterized by rare Hodgkin Reed-Sternberg (HRS) cells surrounded by immune cell infiltrates that define histological subtypes. In 1987, Weiss discovered that a minority of CHL tumors harbored an Epstein-Barr virus (EBV) genome integrated into the genome of the HRS cell (EBV+ tumors) (Weiss, et al., Am J Pathol, 1987). A population-based study conducted in California reported 27% of CHL tumors were EBV+ with a higher prevalence in younger and older patients compared to adolescents and young adults (AYA), and in Hispanics compared to other racial/ethnic groups (Keegan, et al., JCO, 2005). We evaluated EBV tumor status and demographic characteristics and survival in a multiethnic set of CHL patients the Multi Ethnic Study of Hodgkin lymphoma (MESH).
Methods: We collected 891 multiethnic cases of formalin-fixed paraffin-embedded tumor blocks of CHL diagnosed between 1978 and 2018. Patients were identified from cancer centers and hospitals in Atlanta, Los Angeles County, Mexico City, Philadelphia, Detroit, and the Residual Tissue Repositories from Hawai'i and Los Angeles County. CHL diagnosis and histological subtype were confirmed by hematopathologist review. Demographic and clinical information was collected from patient medical charts and state cancer registries. EBV tumor status was assigned based on EBER1 in situ hybridization performed using the Novocastra™ Epstein-Barr virus ISH Kit (Ready-to-use [RTU], Leica Microsystems, Inc. Buffalo Grove, IL) on 2mm cores arranged on tissue microarrays. EBV+ cases were defined by nuclear or cytoplasmic positivity in >5% of HRS cells, determined by a study hematopathologist (IS). We tested associations between EBV tumor status and age at diagnosis (pediatric [<15 years], adolescent/young adult [AYA, 15-39 years], older adults [40+ years]), sex, race/ethnicity (Hispanic, Black, Non-Hispanic White [NHW], Asian, and Hawaiian/Pacific Islander [HPI]), and histology (Nodular Sclerosis [NS]; Mixed Cellularity [MC]; Lymphocyte Rich [LR]; Lymphocyte Depleted [LD]) using χ-squared tests. We also assessed the association between EBV tumor status and overall survival (OS) right censored at 5 years using Kaplan-Meier and multivariable Cox regression analysis adjusted for age at diagnosis (continuous), sex, race/ethnicity, and histology (NS vs. other histologic subtypes).
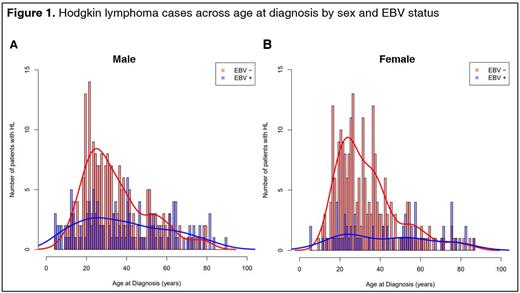
Results: To date, EBV results were available for 638 cases enrolled in the study. Frequency among those with EBV results was as follows: pediatric=45 (7%), AYA=373 (59%), older adults=217 (34%), male=347 (54%), Hispanic=246 (39%), Black=118 (19%), NHW=179 (28%), Asian=60 (9%), and HPI=20 (3%). Overall, 30% of the cases were EBV+. EBV positivity was higher in MC compared to NS cases across all age groups (p=<0.001). By histology and age, EBV positivity for both NS and MC was higher in pediatric (NS [45%]; MC [90%]) and older adult (NS [29%]; MC [64%]), compared to AYA cases (NS [16%]; MC [39%]). The AYA age group comprised the largest number of cases, the majority of which were EBV− (Figure 1). EBV+ cases were more common among males compared to females, with higher prevalence among males of pediatric (62%) and older ages (46%) compared to AYA males (24%), following the same pattern as histology. EBV positivity also differed by race/ethnicity (p=0.002), with the highest prevalence in Hispanics (38%) and lowest in HPI (10%). Survival analyses showed a statistically significantly different OS by EBV status (p<0.001), but after adjusting for age, sex, race/ethnicity, and histology, there was no statistically significant difference in OS between EBV+ and EBV− tumor patients (HR=1.08, p=0.744).
Conclusion: Our results were largely consistent with the previous literature on EBV positivity prevalence in CHL by demographic characteristics. We report low EBV positivity in HPI for the first time, albeit with small numbers in that group. The difference in EBV tumor status by sex is noteworthy, with females having a higher EBV− AYA peak, and males having a higher EBV+ AYA peak. Unlike previous reports, we did not observe an association between EBV status and survival with adjustment for demographic and clinical variables. More studies with diverse populations and larger sample sizes are needed to better understand the complex interplay of EBV patterns in CHL tumors and their significance in different populations.
Disclosures
Allen:Seattle Genetics: Consultancy; Kyowa Kirin: Consultancy; Daiichi Sankyo: Consultancy; Secura Bio: Consultancy. Flowers:Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; Morphosys: Research Funding; Nektar: Research Funding; Novartis: Research Funding; Kite: Research Funding; Jannsen Pharmaceuticals: Research Funding; Iovance: Research Funding; Amgen: Research Funding; Cellectis: Research Funding; Guardant: Research Funding; National Cancer Institute: Research Funding; V Foundation: Research Funding; Karyopharm: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Pharmacyclics Jansen: Consultancy; SeaGen: Consultancy; Spectrum: Consultancy; 4D: Research Funding; Acerta: Research Funding; Genmab: Consultancy; Adaptimmune: Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Genentech Roche: Consultancy, Research Funding; Allogene: Research Funding; CPRIT Scholar in Cancer Research: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; Burroghs Wellcome Fund: Research Funding; Ziopharm: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Pfizer: Research Funding; Xencor: Research Funding; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; TG Therapeutics: Research Funding; Gilead: Consultancy, Research Funding. Scott:Abbvie, AstraZeneca, Incyte: Consultancy; Janssen and Roche: Research Funding. Svoboda:Pharmacyclics: Consultancy, Research Funding; ADCT: Consultancy; Adaptive: Consultancy, Research Funding; TG Therapeutics: Research Funding; BMS: Consultancy, Research Funding; Atara: Consultancy; Astra Zeneca: Consultancy, Research Funding; Genmab: Consultancy; Merck: Research Funding; Incyte: Consultancy, Research Funding; SEAGEN: Consultancy, Research Funding. Steidl:Seattle Genetics, AbbVie, and Bayer: Consultancy; Bristol Myers Squibb, Epizyme and Trillium Therapeutics Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal